Breiftly Explain the Difference Between Permanent and Temporary Hard Water
Permanent hardness is synonymous with noncarbonate hardness. Permanent hardness is simply the hardness that is not removed by boiling.
What Is Temporary Hardness Definition From Corrosionpedia
It is caused due to the dissolved Ca or Mg salts of chlorides or sulphates It can be easily removed by boiling or treatment with lime.

. Hard water ions replace Na or H ions on the resin as follows. Ad Enjoy Clean And Safe Water In Your Home With Our Water Filtration Solutions. To remove the permanent harness usually complex processes such as zeolite or permutit methods are required.
If your water is gypseous that is it has passed through gypsum in the ground it will contain calcium and sulfate. This is how many total divalent ions there are in a given amount of water. Water hardness can be thought of this way.
Boiling and adding lime are two of the older methods used to soften water containing temporary hardness. Carbonate and bicarbonate ions are responsible for this type of water hardnessIt is also known as temporary hardness because it removes from water when we boil the water. Hardness can be removed by boiling water.
What is Hard Water Definition Properties Temporary Hardness and Permanent Hardness 2. Temporary hardness can be largely removed by boiling when bicarbonates are decomposed yielding insoluble carbonates on hydroids. When we boil the water carbonate bicarbonates ions are present in water decomposes insoluble carbonate is reformedBoiling the water.
Temporary hard water is easy to convert to soft water where as permanent hard water is the other way round. Hardness is of two types. Mon Mar 27 173405 2000 Posted by Phil Russell Grade level.
Permanent hard water -- stays hard when boiled temporary hard water -- softened by boiling. That is the main difference between hard water and soft water. Water which does not give lather with soap is hard water.
Hard water also forms scales within the hot water pipes heaters and. Step 1 of 5. The water has a temporary hardness that can be removed by boilingPermanent hardness occurs when the calcium and magnesium salts are not bicarbonates but have another anion like sulfate.
The hardness of water is due to the presence of soluble bicarbonates chlorides and sulfates of calcium and magnesium. It is also known as non-carbonate or non-alkaline hardness. Temporary and permanent hardness are terms used to differentiate between hardness that can be removed by boiling the water temporary from hardness than cannot be removed by boiling permanent.
These can be removed by boiling the water or by treating it with lime. Water containing calcium of magnesium bicarbonates temporary Water containing sulfates of calcium or magnesium permanent Explain how a cation exchange resin softens water. When water is considered temporarily hard its because it contains dissolved bits of calcium and magnesium.
There is a big difference between the temporary and permanent hardness of water. Hardness can be removed by boiling water. Permanent hardness is caused by dissolved calcium sulfate which is not removed by boiling.
Hard water contains dissolved magnesium and calcium ions. Carbonate or Temporary Hardness. C a H C O 3 2 and M g H C O 3 2 makes the water temporary hard water.
Presence of bicarbonates of calcium and magnesium ie. What is the differene between permanent hard water and temporary hard water Date. Soft water is water that has a low mineral content.
Temporary hardness is called as carbonate or alkaline hardness. When you boil water you can get rid of temporary hardness but you cant get rid of permanent hardness. Contains bicarbonate of calcium or magnesium.
Permanent hard water. Permanently hard water on the other hand has calcium and magnesium sulfates that cant be removed with heat and must be treated with a water softener in order to be removed. These make it more difficult for the water to form a lather with soap.
What is Soft Water Definition Preparation 3. Contains sulphates or chlorides of calcium and magnesium. Hard water is water that has a high mineral content.
Temporary hard water. Hard water is the water that requires a large amount of soap to produce lather or foam. Differentiate between the causes of temporary hard water and of permanent hard water.
Hardness of Water - Temporary and Permanent Hardness in Water. Permanent hardness cannot be removed by boiling. When gypseous water is boiled very little hardness is lost because calcium is not.
Temporary hardness is caused by dissolved calcium hydrogencarbonate which is removed by boiling. Temporary hardness is synonymous with carbonate hardness. Water is the most important compound that is needed for the survival of life on earth.
Based on the presence of bicarbonates sulfates and chlorides of calcium and magnesium the permanent water is divided into temporary and permanent hard water. Permanent hardness It is caused due to dissolved Ca or Mg bicarbonates.

What Causes The Temporary And Permanent Hardness Of Water

What Different Methods Are Used For Softening The Hard Water Explain The Principle Used In Each Method Write Any Two

Permanently Temporary Hard Water Causes Removal Of Hardness How To Soften Water Calcium Magnesium Ions Scum Formation With Soap Ion Exchange Washing Soda Gcse Ks4 Science Igcse O Level Chemistry

Determination Of Hardness Of Water By Edta Method Water Engineering Chemistry 1 Youtube

Hard Soft Water Environmental Chemistry Chemistry Fuseschool Youtube
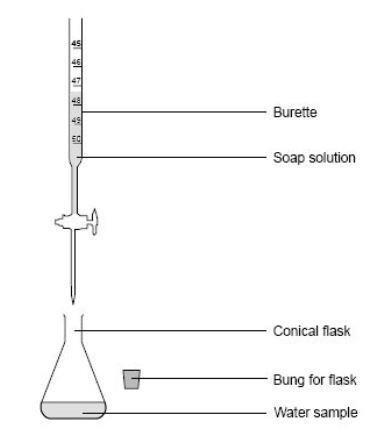
Testing The Hardness Of Water Experiment Rsc Education

Water Hardness Komunalna Dejavnost Bled
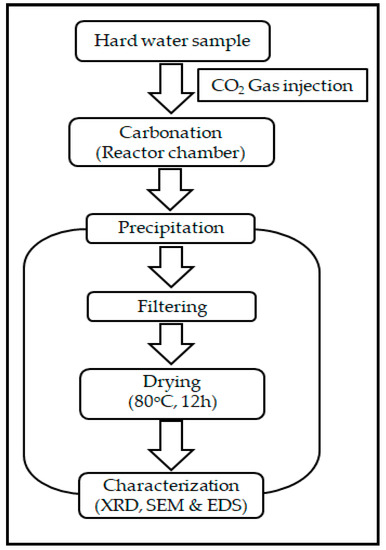
Water Free Full Text Removal Of Hardness From Water Samples By A Carbonation Process With A Closed Pressure Reactor Html
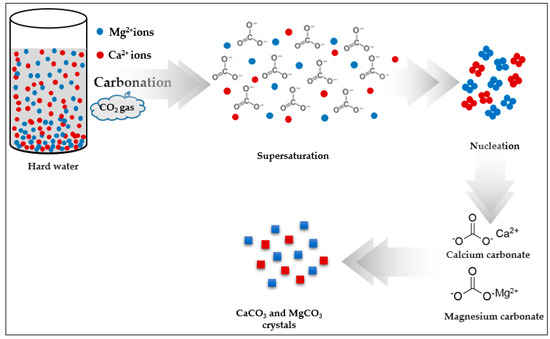
Water Free Full Text Removal Of Hardness From Water Samples By A Carbonation Process With A Closed Pressure Reactor Html
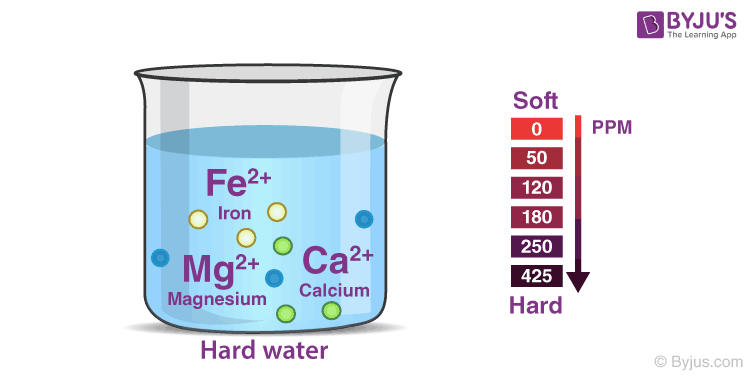
Hardness Of Water Types Remove Temporary And Permanent Hardness

Hardness Of Water Flashcards Quizlet

Tattoo By Saegeem Saegeem Watercolortattoo Watercolor Painterly Fineart Painting Color Monet Flowers Floral Le Small Tattoos Tattoos Popular Tattoos

Learn How To Turn Hard Water Into Soft Water Environmental Chemistry Chemistry Fuseschool Youtube

Difference Between Temporary And Permanent Hardness Of Water Compare The Difference Between Similar Terms

Hardness Of Water Types Remove Temporary And Permanent Hardness

Difference Between Temporary And Permanent Hardness Of Water Compare The Difference Between Similar Terms
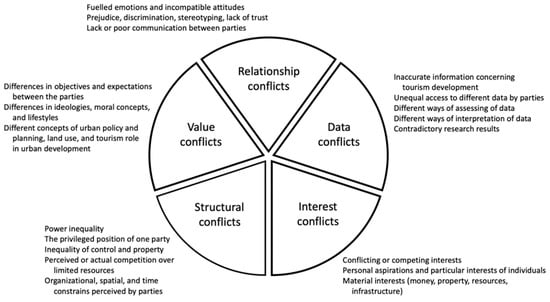
Sustainability Free Full Text Deconstructing The Overtourism Related Social Conflicts Html

Permanently Temporary Hard Water Causes Removal Of Hardness How To Soften Water Calcium Magnesium Ions Scum Formation With Soap Ion Exchange Washing Soda Gcse Ks4 Science Igcse O Level Chemistry

Comments
Post a Comment